In Situ Groundwater Remediation for Complex Mixtures of Inorganic Contaminants
Groundwater remediation is critical for protecting the environment and communities that rely on aquifers as a water resource. Polluted groundwater can be restored by removing contaminants or converting them into harmless substances. Groundwater contaminants may include toxic chemicals, oils, heavy metals, and other potentially harmful substances. Remediation can be performed ex situ (by extracting groundwater and routing it to and above-ground treatment system) or in situ (whereby treatment takes place in the subsurface) to restore groundwater quality to meet state, federal, and local criteria.
Sites contaminated with mixtures of multiple metals, metalloids, and other inorganics can pose unique challenges for in situ groundwater remediation. This is especially true for constituents with differing chemical properties and environmental behaviors, for which effective in situ remediation often requires strategies incorporating combinations of treatment mechanisms to address the specific mixture of contaminants present.
Remediation of Groundwater Impacted by Coal Ash
Coal combustion residuals (CCR) are byproducts of coal-fired power plants throughout the country and have traditionally been stored in ash ponds and landfills. Numerous metals naturally present in coal—including arsenic, boron, cobalt, lithium, mercury, and molybdenum, among others—are concentrated in the coal ash. These metals are often present in soluble forms which can be leached from the ash and potentially contribute to pollution of underlying groundwater. Metals in CCR can include both cationic (positively charged ions) and oxyanion (polyatomic ions that contain oxygen) chemical species, some of which are also redox reactive (subject to reactions that change the oxidation state of the metal). The ionic charge and oxidation state are important characteristics that determine the behavior and reactivity of inorganic contaminants, and are key to identifying chemical reactions that could be engineered to treat impacted water.

Groundwater impacts at CCR facilities can include various metals, metalloids, and other inorganics with widely differing chemical behaviors and treatment requirements.
Different oxide and hydroxide mineral phases have the propensity for taking up and binding cations or anions from water, depending on their crystal structure. In the laboratory, we explored methods to simultaneously synthesize mixed iron-manganese oxides (for cation uptake) and hydrotalcite (a layered double hydroxide of aluminum and magnesium for removal of anions) from solution and tested their effectiveness for concomitant removal of cation and anion species from impacted groundwater collected from coal ash sites. In batch tests, our groundwater treatment removed more than 95% of lithium (cation) and molybdenum (oxyanion), and 60% of boron (oxyanion). Subsequent batch tests focused on optimizing removal efficiencies by tuning the chemistry of the synthesis solutions.
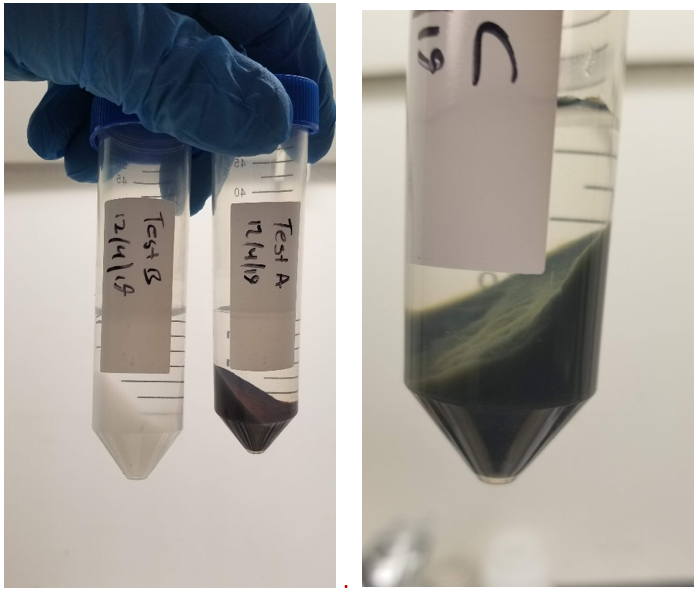
Chemical analysis and XRD confirmed that mixing Mn(II)-Fe(III)-Mg-Cl, an acidic solution, with Na-Al-MnO4, an alkaline solution, was successful in synthesizing precipitates which could simultaneously remove cationic and anionic contaminants.
In Situ Remediation Technique Initiates Chemical Transformation
The in situ remediation technique we explored involves the sequenced injection of two chemical solutions to create a subsurface reactive zone (SRZ). Groundwater treatment occurs via chemical reactions as it flows through the SRZ. Column tests with injection-treated soil and groundwater—which mimicked field conditions— were performed to assess the long-term treatment capacity of the SRZ and the stability of the sequestered contaminants (i.e. their resistant to being remobilized).
When column test results were scaled to hypothetical field conditions, the estimated effective treatment life of a single injection was several years for lithium and molybdenum, but shorter for boron. Therefore, periodic reinjection may be required at some sites. A pilot test now underway will provide field-scale performance data for this in situ reactive zone treatment approach.
Anchor QEA will present the results and key findings of this research on in situ groundwater remediation of complex mixtures of inorganic contaminants during the 2021 Goldschmidt Virtual Conference on Thursday, July 8. Register today for this international conference and our presentation, “Novel Subsurface Reactive Zones Containing Mn-Fe Oxides and Hydrotalcite for In Situ Groundwater Remediation of Multiple Inorganic Contaminants.”


